
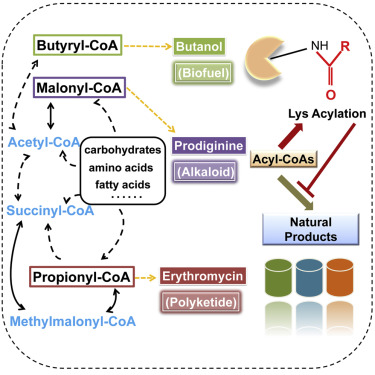
细胞重要中间代谢产物酰基-CoA类化合物,作为供体直接参与生物体内的蛋白酰化修饰,从而调控多种重要生物学过程,如表观遗传、能量代谢、精子发育等,是目前生命科学研究的热点之一。在生物体次级代谢产物生物合成过程中,酰基-CoA扮演的角色一直被认为是聚酮类、生物碱类、脂肪酸类及异戊二烯类等多种重要天然产物的合成前体,然而目前人们对其作为酰化修饰供体调控次级代谢产物合成过程的作用认知明显不足。
中国科学院上海药物研究所谭敏佳课题组与华东理工大学叶邦策课题组合作研究,揭示了蛋白赖氨酸酰化修饰在天然产物的生物合成代谢通路中的调控新机制,研究工作发表在8月Cell Chemical Biology(25(8): 984-995. doi: 10.1016/j.chembiol.2018.05.005)和5月ACS Chemical Biology(13(5):1200-1208. doi: 10.1021/acschembio.7b01068)杂志上。
两篇文章分别以丙酰-CoA依赖性的大环内脂类红霉素、丙二酰-CoA依赖性的多酚类赤松素以及丁酰-CoA依赖性的丁醇生物合成过程中,丙酰化修饰、丙二酰化修饰以及丁酰化修饰为研究对象,通过蛋白质组学技术系统性解析蛋白酰化修饰在不同化学骨架类型的天然产物生物合成过程中的形成机制及调控功能。证明了生物体内高浓度酰基-CoA的积累在有助于补充产物合成前体的同时,也会造成蛋白酰化修饰引起的反馈调控,导致关键酶受到抑制并影响产物产率。
这种由于胞内代谢物浓度的“过载”引起生物体代谢失衡的状态,广泛存在于多种不同化学骨架类型天然产物生物合成过程中,并存在于内源性产物合成途径和人工构建产物合成途径中。
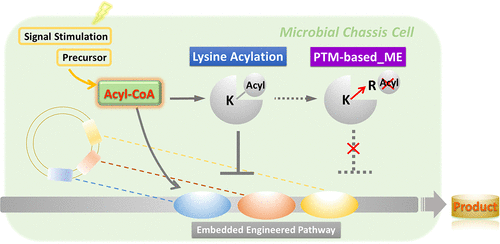
此外,进一步的研究表明,基于酰化修饰底物和修饰酶的翻译后修饰代谢工程策略(PTM_ME),如保护修饰位点、优化修饰酶系统等,有助于缓解胞内碳流“过载”的压力,相对提高目标产物产量。
这两项研究工作首次揭示了蛋白酰化修饰在次生代谢产物生物合成调控中的普遍性,并为代谢工程提供了从翻译后修饰水平改造的全新策略。(来源:生物通)
Protein Acylation is a General Regulatory Mechanism in Biosynthetic Pathway of Acyl-CoA-Derived Natural Products
Abstract Coenzyme A (CoA) esters of short fatty acids (acyl-CoAs) function as key precursors for the biosynthesis of various natural products and the dominant donors for lysine acylation. Herein, we investigated the functional interplay between beneficial and adverse effects of acyl-CoA supplements on the production of acyl-CoA-derived natural products in microorganisms by using erythromycin-biosynthesized Saccharopolyspora erythraea as a model: accumulation of propionyl-CoA benefited erythromycin biosynthesis, but lysine propionylation inhibited the activities of important enzymes involved in biosynthetic pathways of erythromycin. The results showed that the overexpression of NAD+-dependent deacylase could circumvent the inhibitory effects of high acyl-CoA concentrations. In addition, we demonstrated the similar lysine acylation mechanism in other acyl-CoA-derived natural product biosynthesis, such as malonyl-CoA-derived alkaloid and butyryl-CoA-derived bioalcohol. These observations systematically uncovered the important role of protein acylation on interaction between the accumulation of high concentrations of acyl-CoAs and the efficiency of their use in metabolic pathways.
原文链接:https://www.cell.com/cell-chemical-biology/pdf/S2451-9456(18)30152-1.pdf
Protein Acylation Affects the Artificial Biosynthetic Pathway for Pinosylvin Production in Engineered E. coli
Abstract The effect of regulatory system on the engineered biosynthetic pathway in chassis cells remains incompletely understood in microorganisms. Acyl-CoAs function as key precursors for the biosynthesis of various natural products and the dominant donors for protein acylation. The polyphenol pinosylvin, with high antimicrobial and antifungal activities, is biosynthesized with malonyl-CoA as its direct precursors. But correlation between lysine malonylation and pinosylvin biosynthesis remains unknown. Herein, we found that the malonyl-CoA-driven lysine malonylation plays an important role in interaction between the engineered pathway of pinosylvin synthesis and E. coli chassis cell. Oversupply of malonyl-CoA leads to an increase in malonylation level of global proteome as well as the enzymes in the artificial pathway, thereby decreasing yield of pinosylvin. The results revealed that the intricate balance of cellular acyl-CoA concentrations is critical for the yields of acyl-CoA-derived natural products. We next modified the enzymes in the biosynthetic pathway to adjust their acylation level and successfully improved the yield of pinosylvin. Our study uncovers the effect of protein acylation on the biosynthetic pathway, helps optimization of synthetic constructs, and provides new strategies in metabolic engineering and synthetic biology at the protein post-translational level.
原文链接:https://pubs.acs.org/doi/pdf/10.1021/acschembio.7b01068



